Garcinia Cambogia Extract Tablets: A Natural Fat-reducing Raw Material
 2025-08-14 11:02:25
2025-08-14 11:02:25
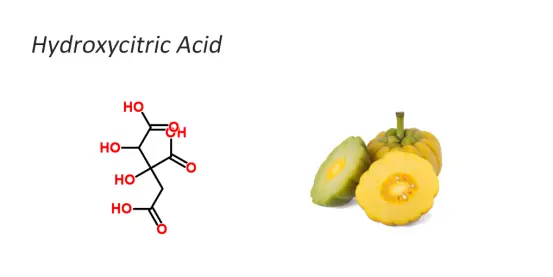
Hydroxycitric acid is a derivative of citric acid and is widely present in tropical rainforest plants such as garcinia cambogia and roselle. garcinia cambogia is a kind of tree in the family Baricaceae of the class Dicotyledonous plants, also known as Malabarophora. Its fruit is yellow and shares the same name as the plant species. The fruit is almost the same size as an orange and its outer surface is very similar to that of a pumpkin. The yellow vine fruit grows in the damp and dense forests on low slopes. Its native habitat is South Asia. For centuries, it has been widely cultivated in southern India and Thailand as a fruit-shaped herb. Biological health products extracted from the fruits of the rattan vine have become popular all over the world. Garcinia Cambogia Extract Tablets (Hydroxycitric acid)has the effect of inhibiting certain enzyme reactions and reducing appetite. It is said that eating rattan vine fruits before each meal can help lose 1 pound of weight every day.
The Discovery of Garcinia Cambogia Extract Tablets
Hydroxycitric acid is present in large quantities in the peels of certain wisteria fruits that grow in the Indian subcontinent and Sri Lanka. The fruit of Cambogia, after being dried in the sun, is called Marabar tamarind fruit. It is widely used for cooking purposes and fish pickling on the west coast of South India. The organic acids in the fruits exert antibacterial effects in the pickling liquid by lowering the pH value. This fruit contains 30% acid (calculated by the dry basis weight of citric acid), which was previously mistaken for tartaric acid and citric acid. When the water extract of this acid was developed on paper chromatography using different solvents, two main acid spots appeared, which were quite close to the spots of tartaric acid and citric acid. However, the significant difference in Rf values was very small in all solvent systems. When Palmer's ion-exchange column method was used to analyze the juice, the maximum peak appeared in the region corresponding to citric acid. However, this acid did not appear when tartaric acid was determined in the tartaric acid test or when citric acid was determined in the pentabromoacetone test.
Lewis et al. isolated this major acid from the peel of Cambogia rattan and based on fundamental chemical and optical research that is designated as HCA. Use n-butanol/acetic acid/water (4:1:5) and n-propanol/formic acid/water (4:1:5) solvents on Whatman No. 1 filter paper,the system identifies and separates HCA. The location of the spots was identified by spraying 5% vanadate. Once the acid is mixed with an excess of alkali,The compound was saponified and loaded onto an ion-exchange resin column (Zeocarb215), with only one spot (Rf) appearing in the eluent at a lower position
=0.34), which corresponds to the free (-)-HCA; Once concentrated, only one spot (Rf=0.46) appears in the eluent at a higher position.It corresponds to (-)-HCA lactone.
When elution was carried out using two different solvent systems, spots of two main acids appeared on the spectrum of the fruit extract. Once Titrate the substance with alkali. When phenolphthalein is used, two different ends appear after cooling and boiling, which is manifested as lactone
Characteristics. The two spots on the spectrum have been identified as hydroxycitric acid and its lactone. It is quite clear that these two spots on the chromatogram correspond to the positions of Y-hydroxy acid and lactone, rather than tartaric acid and citric acid.
Separation and Preparation of Hydroxycitric Acid
Lewis et al. isolated hydroxycitric acid on a large scale from the peel of Cambogia rattan. This method consists of the following steps: under reduced pressure (10lb/in ₂,15min), the raw materials are boiled in water to extract the acid. After the extract is concentrated, the pectin is removed by ethanol precipitation. The filtrate is neutralized with alkali to clarify the filtrate, and the acid is recovered through a cation exchange resin column. The acid is then concentrated and dried to obtain the crude product. The dried crude product was then extracted with ether and recrystallized to obtain small needle-shaped crystals - lactones. Lewis also reported another method for separating hydroxycitric acid from Cambogia wisteria using acetone. The acetone extract was concentrated, and the acid was absorbed by water. Once the aqueous solution was evaporated, the acetone was recovered.
Moffett et al. proposed a process for extracting hydroxy citric acid from the aqueous solution of the peel of rattan yellow fruit, and the extract was placed on an anion exchange tree,the lipid column is used to adsorb (-)-HCA, which is then eluted with NaOH/KOH to release (-)-HCA. Free acid ° was obtained from the extract of the vine yellow fruit by cation exchange resin column. Guthrie et al. and Moffett et al. respectively reported the methods for preparing hydroxy citric acid concentrate from the peel of the vine yellow fruit.Among them, the content of HCA is 23% to 54%, and the content of lactone is 6% to 20%.
The Efficacy of Garcinia Cambogia Extract Tablets
1. Regulate the level of fat metabolism in organisms
Laboratory and animal experiment results suggest that HCA may have the ability to regulate the level of fat metabolism in organisms. HCA has thus become one of the ingredients in some weight loss products and nutritional health supplements.
One of the HCA isomers :(2S,3R)-HCA, has the effect of inhibiting pancreatic α -amylase and small intestinal α -glucosidase, leading to a decrease in in vitro glucose metabolism levels (reducing glucose absorption and utilization).
A hydroxycitric acid (HCA) with a similar structure to citric acid was discovered from the fruit of caciniaC ambigia, a plant in Southeast Asia. HCA has the effect of inhibiting certain enzymatic reactions and reducing appetite. Eating this fruit before each meal can help lose 1 pound of weight every day. Similar to 5-hydroxytryptamine (5-HTP), which can suppress appetite and help with weight loss, is a precursor of the neurotransmitter 5-hydroxytryptamine. For overweight women, taking 600 to 900mg of 5-HTP daily can lead to significant weight loss.
2. Dissolve calcium oxalate-type kidney stones
The latest research suggests that hydroxycitric acid can help dissolve calcium oxalate crystals, which are the main components of kidney stones. Jeffrey Rimer, an assistant professor at the University of Houston, claimed that this discovery represents the first significant breakthrough in the treatment of kidney stones in over three decades. His research has been published in the journal Nature. Studies have proved that hydroxycitrate can not only prevent the formation of calcium oxalate crystals, but also dissolve the already formed stones. Over the past 30 years, there has been almost no progress in the preventive treatment of kidney stones. Doctors tell patients to avoid foods with high oxalic acid content and to take citrate, but side effects often occur.
From a chemical structure perspective, hydroxycitrate only has one more "hydroxyl group" than citrate. Although it only has one more hydroxyl group, hydroxycitrate is more effective in preventing stones than citric acid, making it a new therapeutic drug. The story behind this discovery might be that the research team used hydroxycitrate as a citrate control drug. The powerful effect of dissolving stones was discovered by chance, and hydroxycitrate is also a natural plant compound, suggesting that an ideal therapeutic drug for kidney stones may have been found.
In addition, scientists have found that when rats were fed hydroxycitrate, the rate of gluconeogenesis (the formation of glucose and glycogen in the liver) was almost twice that of normal rats. Therefore, promoting gluconeogenesis can increase the feeling of fullness. This means that supplementing with hydroxycitrate can enable animals to feel full by eating less food, and at the same time help accelerate the consumption of excess fat in the body. Studies show that while hydroxycitrate increases fat consumption, the oxidation rate of proteins slows down. As a result, when the diet was supplemented with hydroxycitric acid to reduce weight, the loss of muscle tissue was very little. Obese patients are also prone to hypertension, cardiovascular diseases, diabetes and some other diseases. Hydroxycitric acid can inhibit the pathogenic factors by reducing fat.

Summary
To sum up, when people consume sugars, these sugars are broken down into glucose, transported to the muscles and converted into calories. However, when the intake of calories exceeds the expenditure, the excess glucose will be converted into fat and stored, which is the cause of obesity. If hydroxycitric acid is taken before meals, it can prevent glucose from being converted into fat and promote its conversion into glycogen, which is more easily utilized as energy. Therefore, it can prevent obesity. In addition, hydroxycitric acid can stimulate the satiety center, making it less likely for the human body to feel empty, and thus the amount of food intake will naturally decrease. Moreover, hydroxycitric acid has no other side effects and is less likely to cause weight rebound after losing weight. With proper dietary control and physical exercise, taking hydroxycitrate has a significant effect on weight loss. Therefore, the fruit of the vine yellow and its extracts are the most popular weight loss health food in the United States.










_1756176543590.webp)
_1747986913239.webp)
_副本_1753942900004.webp)
_副本_1753953089833.webp)

_1754470372778.webp)
_1755068906494.webp)


